NMR CLINICAL RESEARCH SOLUTIONS
Advance your metabolomics research of human biofluids with a push-button, standardized and fully automated, nuclear magnetic resonance (NMR) solution
Furthering human disease research
with powerful technology
Enabling Standardization of IVDr by NMR: Using Bruker IVDr Methods for Urine/ Plasma/ Serum Quantification.
Clinical and translational research by NMR is rapidly growing and worldwide networks of research groups such as the Phenome Center Network are forming. As a result, there is an increasing focus on standardization to maintain the transferability and reproducibility of the data.
Currently there is an emphasis to standardize analytical instrumentation, operation procedures, analysis procedures and metadata handling
Now available on the AVANCE™ IVDr system, B.I.-Methods is the basis for data analysis technology developments and enables standardization of body fluid analysis by NMR.
B.I.-Methods link the IVDr platform with Bruker’s automatic data analysis service and contains all automation methods needed for standardized data generation of body fluids. It also includes efficient quality control methods needed when working on human samples and ensures transferability of spectral data generated. While the entire system is for research use only, B.I.-Methods paves the way for possible future use in clinical settings by fulfilling certain requirements for accreditation and certification.
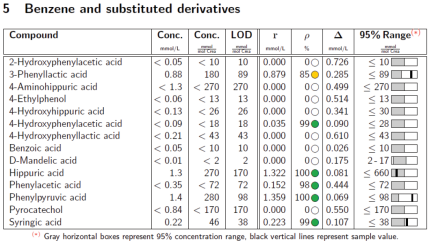
Analysis solution that complies with DIN-ISO criteria for the identification and quantification of urine metabolites (for research use only)
Urine metabolic analysis is particularly valuable and information-rich, because the metabolic pathways of a wide range of nutrients, drugs and environmental contaminants can be identified in urine, even if urine is very complex to analyze. Large sample cohorts in clinical studies demand automated solutions capable of measuring and analyzing with highest information content and reproducibility.
The B.I.QUANT-UR™ module of Bruker’s successful Avance® IVDr (In Vitro Diagnostics research) platform now provides precise, sensitive and fully reproducible results that have demonstrated great potential in clinical/translational research.
With the new version B.I.QUANT-UR 1.1, routines and validation parameters have been improved and enables now more positive hits. The combination of raw concentration with the quality assessment parameters available allow to obtain reliable quantification results even below LOD.
New plasma and serum quantification package , which detects and quantifies up to 40 different metabolites .
The Bruker IVDr platform is optimized towards supporting the analysis of epidemiological studies with large scale, dedicated clinical and translational trials, and high throughput Biobank quality control. Based on nuclear magnetic resonance (NMR) spectroscopy, the standardized Bruker IVDr Methods module (B.I. Methods) guarantees high reproducibility and transferability under full automation at anytime and anywhere what is not achievable by any other analytical tool.
This standardized platform allows to combine different solutions. For plasma/serum, it means Bruker IVDr Lipoprotein Subclass Analysis (B.I.LISA™) and automated quantification of small molecule metabolites (B.I.QUANT-PS™) using the same experiment.
B.I.QUANT-PS 2.0 offers the quantification from 26 metabolites up to 41 metabolites.
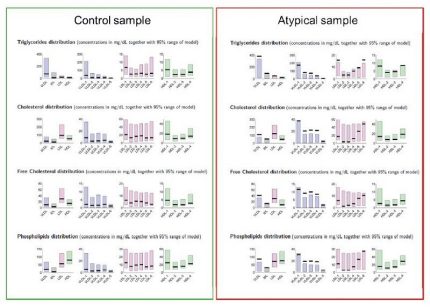
Lipoprotein Subclass Analysis for cardiovascular risk assessment(for research use only)
Lipoproteins are supramolecular assemblies that transport water-insoluble lipids in blood. Lipoproteins also carry apolipoproteins, which determine structure and
function. Apolipoproteins, a monolayer of amphiphilic phospholipids and cholesterol embedded therein, constitute the surface of the lipoproteins that 'hide' the lipids from the aqueous surrounding. The inner core mainly consists of triglycerides and esterified cholesterol.
In the context of cardiovascular risk assessment, the lipoprotein class-specific concentrations of cholesterol and triglycerides are of interest beyond their total concentrations within the plasma. There is a strong indication that additional sub-classification of lipoproteins could improve cardiovascular risk prediction.
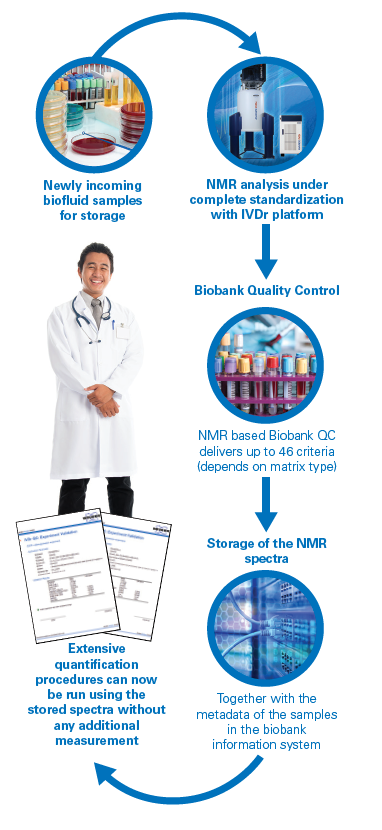
While the number of Biobanks worldwide is growing rapidly, quality control of the whole process Is a requirement to ensure the value of the biobanks. Standardization is needed to allow researchers to integrate results obtained of specimen tests from 1 or more biobanks. Standardization also includes the QC Process, which needs to cover all aspects of pre-analytics and sample storage. In addition validation of specimen/donor metaparameters is of additional value.
NMR is especially suited to perform QC-analysis of liquid biopsies and can deliver a large number of criteria based on one QC measurement per sample. In addition to QC Information NMR can deliver a large number of metabolic information using the same spectra generated during the QC process. With this information in urine 150 metabolites in 2 age ranges are quantified. In plasma/serum 115 lipoprotein related parameters (including subclasses) and 26 metabolites/parameters are analyzed and quantified, the whole process is under push button automation and can be handled by a trained medical technical assistant.
In addition to quality control, biobanks can now support epidemiological studies or clinical trials by using the metadata of selected NMR spectra or quantification results. This will reduce the need for new aliquots and substantially reducing the cost of clinical trials or research studies.
Further savings can be gained by sharing the spectral data. New NMR-based diagnostic tests can be validated on a worldwide basis and on multiple phenotypes without exploding the cost of the trial. Data generated from an 11 IVDr platform Inter-Laboratory Trial proves this unique benefit of NMR1.
Bruker IVDr for NewbornScreening (B.I.QUANT-UR ne)
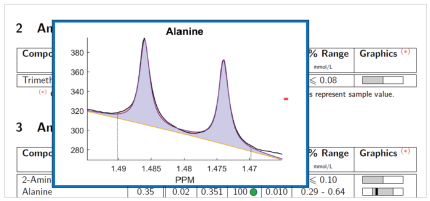
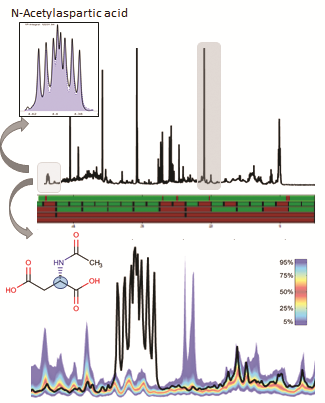
Urine selective screening by NMR combines targeted analysis of metabolites including Inborn Error of Metabolism and other disease markers and non-targeted classification against healthy newborn models. Non-targeted screening enables the detection of all NMR-visible deviations from normal references, whether known or unknown. Figure 1 illustrates the identification of deviations from normality. All NMR spectra included inthereference model are combined in a so-called quantile plot, shown as a color band over the NMR spectrum. A spectrum from a new sample can be overlaid and tested for consistency with the model, i.e. all resonances will fit into the envelope defined by the model. This testing can be performed automatically in a uni- or multivariate fashion.The sample spectrum represents a canavan disease case. In the expansion of the overallNMR spectrum, signals of N-Acetylaspartic acidare clearly visible. As part of the B.I.QUANT-URpanel, N-Acetylaspartic acid can be identified andquantified automatically; however statistics would also reveal the existence of previously unseen deviations with the same certainty.
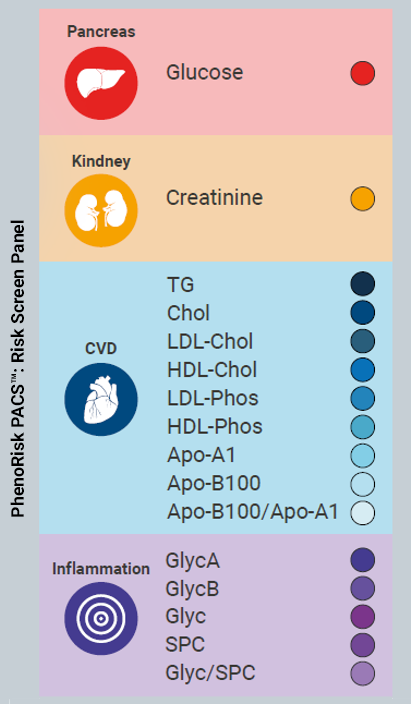
New plasma/serum quantification package detects and quantifies 16 different metabolites and ratios thereof in the context of research in COVID and the Post-Acute COVID-19 Syndrome (PACS). It indicates the potential for providing personalized risk information related to cardiovascular disorders, Type II Diabetes, kidney disorders and the overall inflammatory status.
PhenoRisk PACS™ enables highly reproducible quantitative multiplexed testing supporting researchers in the early detection of clinically well-characterized risk markers.
PhenoRisk PACS™ is a RuO solution for Bruker’s standardized and automated Avance IVDr NMR platform enabling researchers in-depth characterization of pathomechanisms of SARS-CoV-2, even in asymptomatic individuals, thus having the potential the potential to acting as a multi-organ risk screening for organ dysfunction after SARS-CoV-2 infection in a single laboratory test.
PhenoRisk PACS™ might enables the study of SARS-CoV-2 triggered metabolic phenoconversion, defined as transient or persistent systemic change of the molecular signature in human blood after acute SARS-CoV-2 infection. Subsequent phenoreversion, indicated by normalization of the metabolic signature, can be detected by PhenoRisk PACS™ and may mark PACS recovery.
PhenoRisk PACS™ provides insight into unique (RuO) biomarkers, exclusively assessable by NMR technology (Glyc A/B, SPC) for deeper information on ongoing inflammation processes.
PhenoRisk PACS™ deepens the understanding of the multisystemic nature of SARS-CoV-2 and PACS thus supporting basic and translational clinical research as well as the development of impactful SARS-CoV-2 treatments by:
Metabolite Reference Database
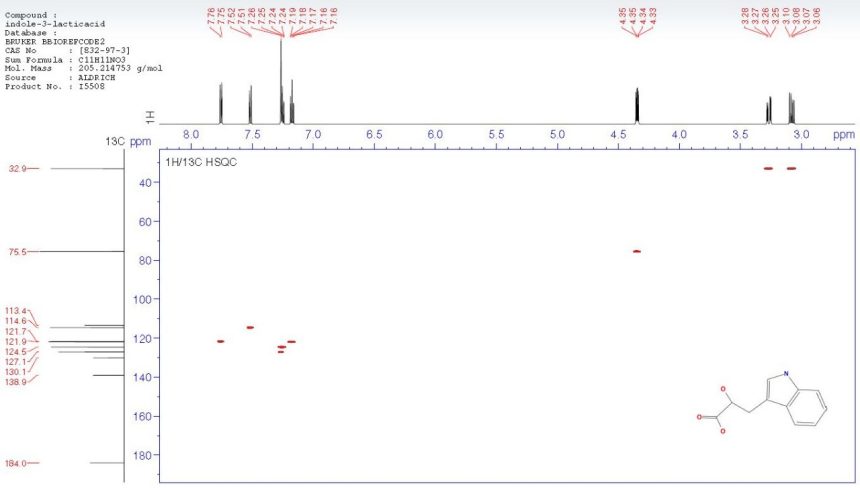
The BBIOREFCODE 2 is a database containing currently spectra of 800 compounds which are typically found as metabolites in body fluids or as components in samples from food, feed and beverage analysis. In addition also a variety of typical contaminants is contained.
AMIX Software provides powerful search algorithms to identify the compounds in the database in mixture spectra. This allows the assignment of unexpected signals in spectra from screening applications and the identification of the components.
Spectra are supplied at different pH values so that experimental conditions of mixture spectra and database can be matched for database search.