NMR & EPR Pharma Solutions
NMR-powered solutions with a wealth of structural information at physiological conditions
Overview
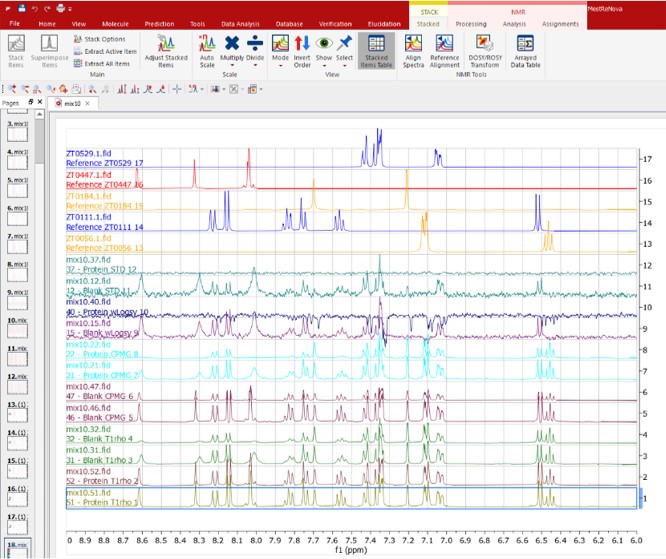
Complete workflow from fragment library quality check, cocktail design and automated hit identification to quantitative assessment of binding (Kd) and the 3D analysis of ligands.
Designed to monitor reactions, enhance reaction understanding, gain confidence when transferring processes from lab to plant.
InsightMR Flow Unit
Design for on-line reaction monitoring by NMR, InsightMR flow unit enables simultaneous acquisition of data using different analytical techniques (NMR, IR, pH, and MS) and captures the start of the reaction.
Enabling the monitoring of living cells, and enhancing understanding of their biological activity, through direct measurement of metabolites concentration variations.
InsightCell is based on established InsightMR™ technology, originally developed for monitoring chemical reactions on-line. The flow unit and software, however, can now be used for monitoring living cells.
There are several approaches for monitoring metabolites in biological processes. For example, cells can be fed with nutrients in the flow tube, or the culture can flow from the bio-reactor to the NMR spectrometer.
The dynamic monitoring of bioprocesses is useful for understanding reactions and optimizing processes. Fermentation processes can be easily monitored using NMR, by measuring the increasing concentration of ethanol in the mixture.
InsightCell enables the optimization and control of conditions such as pH and temperature, which are critical factors affecting the yield of bioprocesses.* The principle can be applied to the production of antibiotics, vaccines and vitamins alike.
Fastest of the Insight product line and ideal for rapid reaction optimization and monitoring of fast reactions magnetic resonance
InsightXpress™ is the fastest of the Insight product line and ideal for rapid reaction optimization and monitoring of fast reactions MR.
InsightXpress’s new stop-flow delivery pumps enable reaction conditions to be screened by NMR at an unprecedented speed.
Quantitative NMR, direct efficent solution for potency determination. Now also under GxP!
Potency determination, absolute compound purity assessment, identity testing, residual solvent, moisture analysis, relative response factor calculation... Have you thought about using quantitative NMR as a one-stop solution?
Potency determination by qNMR has been shown to be a single point replacement for routine development testing which previously involved several experiments and techniques[1] and a superior method for determining the purity of the reference standards[2] used for analysis with assured metrological traceability[3] such as standards for HPLC.
The downside is that existing qNMR workflows rely on expert knowledge and/or well-trained analysts and detailed protocols. Bruker-Mestrelab new tools for potency/purity determination streamline this process with a fully automatic workflow from experiment submission to report, making it the ideal solution for both experts and non-experts working in a pharmaceutical development environment, where quality is a must.
Easy-to-use benchtop system meets the analytical power of NMR to determine the ratio of either different drug components or various solid forms in a substance.
Quantitation of components in solid mixtures like formulated materials is crucial in material sciences. Especially in the pharmaceutical industry, API (Active Pharmaceutical Ingredient) form characterization and quantification play a central role from early drug development to manufacturing, including knowing how the presence of these solid forms is influenced by the production and storage of drugs, as well as interactions with excipients. Current API quantification methods can be expensive, require laborious calibrations or lack accuracy.
Bruker makes use of reliable and affordable TD-NMR (Time-Domain Nuclear Magnetic Resonance) to monitor phase purity and quantify physical API forms including amorphization. The patent pending minispec Form Check uses 1H or 19F relaxometry data as fingerprints for expected components in solid mixtures, superseding excessive calibration, delicate sample preparation and a high level of expert knowledge.
Magnetic resonance in the characterization and quantification of polymorphs
Although magnetic resonance is considered a well-established powerful technique for studying polymorphs and amorphous forms, thus far it has resided in the hands of few specialists. Recent technological advances, however, have enabled the deployment of the technique to a much wider scientific audience. We have overcome the price and complexity barriers, with the introduction of a benchtop spectrometer and have greatly increased automation and sensitivity with our high-resolution floor standing instruments.
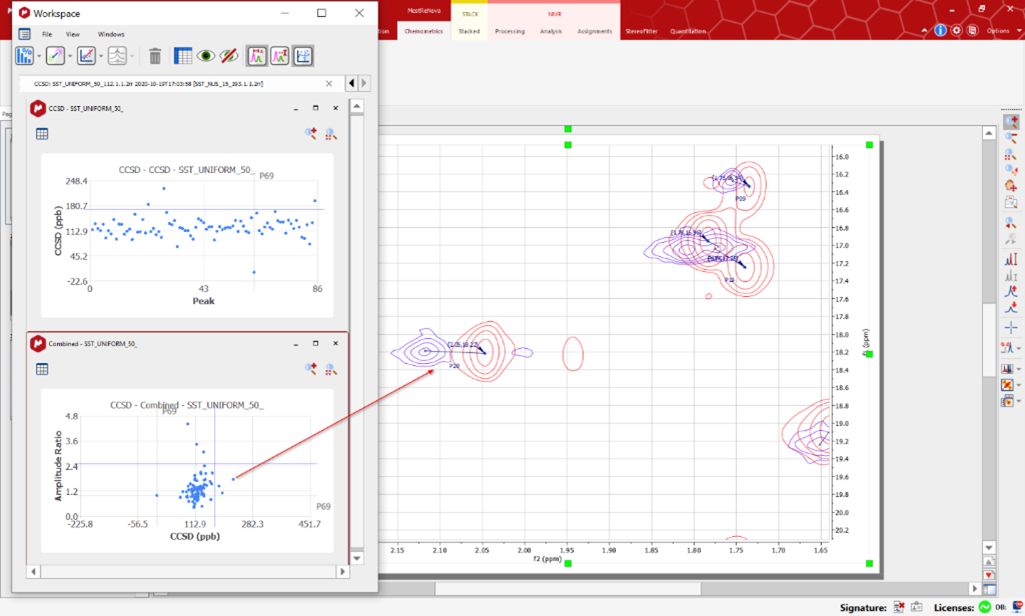
Evaluating the Higher Order Structure (HOS) of an intact protein/antibody in physiologically relevant buffer. Small, but relevant changes in the HOS are detected at atomic resolution.
The latest generation of NMR instrumentation, combined with newly developed NMR acquisition methods, means that intact molecules, including antibodies (150 kDa), can be characterized at natural abundance, in formulation buffer or other physiologically relevant conditions. Differences between batches are detected by comparing the spectrum of a batch with a reference, thus allowing rapid pass/fail decisions to be made.
Established TD-NMR methods are now back in the spotlight with the rise of biotherapeutics.
High accuracy, 100% fill check of vials and syringes is now possible in a matter of seconds. The method is non invasive, preserving the sterility of the samples, and it comes in an affordable benchtop format!
Contactless Check Weighing (CCW) by the minispec is attracting more and more attention because of its matchless advantages for pharmaceutical production giving rise to optimized control of the filling lines. The benchtop minispec mq CCW utilizes TD-NMR, a magnetic resonance technology similar to MRI.
The pharmaceutical product is filled into syringes, vials or ampoules to a certain mass or volume, a quantity which has to be carefully controlled. The common method is weighing via a balance, which, for obvious reasons, is a two-step process.
GxP enabling kit with new software tools specially designed for state-of-the-art data integrity and compliance according to 21 CFR, part 11 principles.
New tools for Data Integrity (DI), compliance and good laboratory and manufacturing practices.
Controlling chemical reactions with artificial intelligence
The PIPAc Project
The companies NovAliX, Alysophil, De Dietrich Process Systems and Bruker have joined forces to bring to market a new approach to active pharmaceutical ingredient (API) production. The partnership will leverage its complementary skills to provide a complete, standalone, and location-independent API manufacturing solution to a pharmaceutical company or contract manufacturing organization.